Deregulation of MYC genes occurs in up to 70% of all human cancers and is associated with hallmarks of cancer including mitochondrial and ribosomal biogenesis, cell cycle progression, and metabolic abnormalities. TP53 regulates MYC while MYC suppresses TP53, suggesting counteracting negative feedback loops. Therefore, MYC or its function can be activated when TP53 is not functional. TP53 mutations occur in 30% of relapsed/refractory acute myeloid leukemias (AMLs) patients' survival is dismal, and there are no effective therapies for these patients. Compared to TP53 wild-type (TP53wt), TP53 mutant (TP53mut) AMLs have lower percentages and numbers of leukemia blasts with increased immature CD34+ cells and resistance to chemo- or molecularly targeted therapies. However, the exact cellular hierarchy of TP53mut AML has not been elucidated.
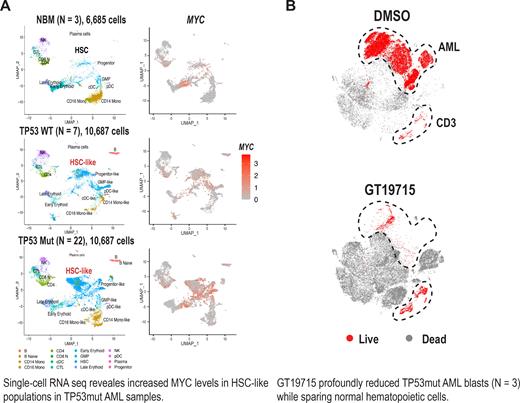
We observed significantly increased MYC mRNA levels in (TP53mut), as compared to TP53wt AML, and also increased levels in TP53mut versus TP53wt AML leukemia stem cell (LSC) fractions. This finding was confirmed in a dataset from the Munich Leukemia Laboratory (N = 732). We found significantly upregulated MYC pathways in TP53mut compared to TP53wt AML LSC. We confirmed the increased MYC mRNA levels at the protein level in TP53mut AML by single-cell mass cytometry (CyTOF). To dissect the cellular hierarchy in TP53mut AML, we performed single-cell RNA sequencing of 32 BM samples from healthy donors (N = 3), newly diagnosed, high-risk TP53wt (N = 7) and TP53mut (N = 22) AML patients, with 6,685, 10,687 and 10,687 cells from normal, TP53wt and TP53mut AML bone marrow (BM) cells, respectively. We found highly enriched HSC-like cells and reduced progenitor- and GMP-like cells in TP53mut AML compared to normal BM (NBM) and TP53wt AML samples. We overlayed MYC expression levels on the mapping of cellular components and found higher MYC levels in HSC-like cells in TP53mut AML compared to HSC and HSC-like cells in NBM and TP53wt AML samples, suggesting enrichment of immature HSC-like cells and increased activity of MYC in TP53mut AML LSCs ( Fig. A).
To target c-MYC and MYC signaling, we utilized GT19715, the first-in-class cereblon modulator (CELMoD) for c-MYC protein (Nishida, ASH 2022). CyTOF confirmed the presence of much increased c-MYC protein levels in primary CD34+ AML than in CD34+ NBM hematopoietic stem cells (HSCs). Notably, CD34+ AML cells showed much greater sensitivity to GT19715 compared to CD34+ NBM cells. Data suggest on-target activity of GT19715 against c-MYC in LSCs with a therapeutic window between LSCs vs. NBM HSCs. Intriguingly, c-MYC protein levels are higher in CD34+CD38+ than in CD34+CD38- AML cells, suggesting that c-MYC drives the proliferation of AML progenitor cells differentiating from quiescent LSCs. Consequently, CD34+CD38+ AML progenitor cells exhibited greater sensitivity to GT19715 compared to CD34+CD38- LSCs. Using paired, TP53 null HL-60 GT19715-sensitive and -resistant cells generated through chronic exposure to GT19715, we interrogated the impact of GT19715 on metabolic changes. GT19715 induced pronounced reductions in basal and maximal oxygen consumption rates (OCRs) in GT19715-sensitive HL-60 cells. GT19715 reduced both basal, glutamine- and glucose-dependent extracellular acidification rates (ECARs) in GT19715-sensitive cells while no inhibition in OCRs or ECARs was observed in GT19715-resistant cells. GT19715 severely inhibited ECARs even after adding glucose to GT19715-sensitive cells, suggesting irreversible inhibition of glycolysis as one of the mechanisms of action of GT19715. GT19715 profoundly reduced AML blasts in TP53mut AML samples (N = 3) ( Fig. B), and in a very aggressive patient-derived xenograft (PDX) TP53mut AML model established from a patient with TP53 p.Y220C and p.P151A mutations along with MECOM rearrangement and K/NRAS mutations. In humanized Crbn I391V mice, where the Crbn-mediated protein degradation is operational, GT19715 only reduced WBC counts along with minimal body weight loss. GT19715 but did not reduce total mouse BM CD45+ cells, suggesting favorable toxicity profiles of GT19715.
In conclusion, TP53mut AML comprised highly enriched LSC populations compared to TP53wt AML and targeting of c-MYC protein is highly effective in TP53mut AML in vitro and in vivo with a therapeutic window between AML LSC and normal hematopoietic cells.
Disclosures
Nishida:Kintor Pharmaceutical: Research Funding. Carter:PinotBio: Research Funding; PMV: Research Funding; Revolution Medicines: Research Funding; Syndax: Research Funding. Maiti:Celgene: Research Funding; Lin BioScience: Research Funding. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Ren:Kintor Pharmaceutical: Current Employment. Tong:Kintor Pharmaceutical Ltd: Current Employment, Current equity holder in publicly-traded company. Chen:Kintor Pharmaceutical: Current Employment. Issa:Celgene: Research Funding; Syndax: Research Funding; NuProbe: Consultancy; Novartis: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding; Merck: Research Funding. Andreeff:Kintor Pharmaceutical: Research Funding; PMV: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal